Mobile Menu
-
Our Scientists
- Andrews, Brenda
- Angers, Stephane
- Attisano, Liliana
- Babaian, Artem
- Bader, Gary
- Blencowe, Benjamin
- Boone, Charles
- Brown, Grant
- Chan, Warren
- Fraser, Andrew
- Friesen, James (Professor Emeritus)
- Gilbert, Penney
- Gillis, Jesse
- Goeva, Aleksandrina
- Greenblatt, Jack
- Harrington, Lea
- Hughes, Timothy
- Kim, Philip M.
- Krause, Henry
- Montenegro Burke, Rafael (Rafa)
- Morris, John
- Morshead, Cindi
- Radisic, Milica
- Röst, Hannes
- Roth, Frederick (Fritz)
- Roy, Peter
- Ryu, William
- Sefton, Michael
- Shoichet, Molly
- Stagljar, Igor
- Taipale, Mikko
- van der Kooy, Derek
- Wang, Shu
- Wheeler, Aaron
- Yip, Christopher
- Zhang, Zhaolei
- Research
- Platforms
- Students
- Postdocs
- News
- Careers
- Events
- About Us

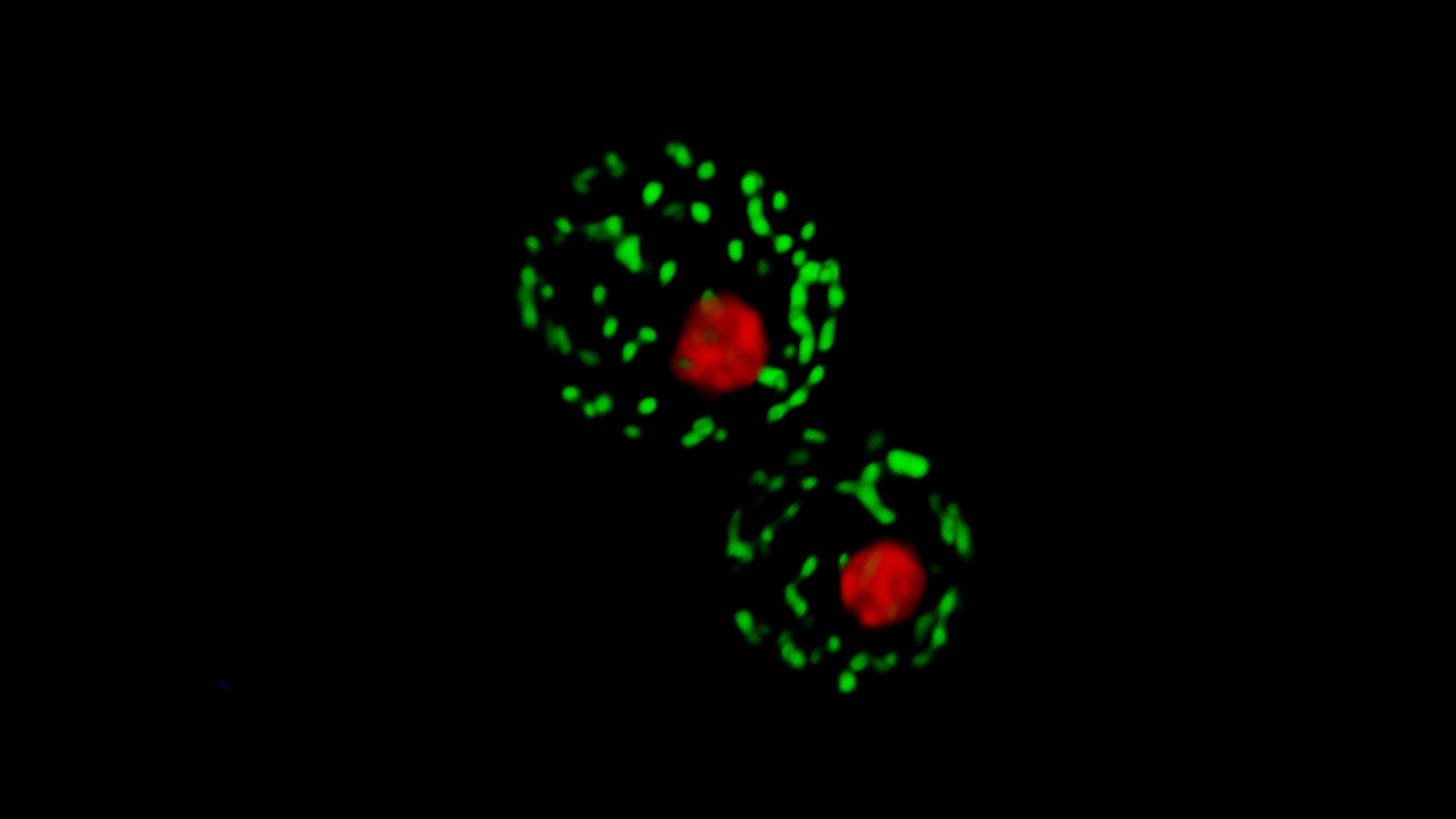
 By testing the effects of human mutations in yeast, Roth’s research team at the University of Toronto’s Donnelly Centre and Lunenfeld-Tanenbaum Institute was able to identify harmful changes in the DNA better than leading algorithms. The ultimate goal of his approach, detailed in the
By testing the effects of human mutations in yeast, Roth’s research team at the University of Toronto’s Donnelly Centre and Lunenfeld-Tanenbaum Institute was able to identify harmful changes in the DNA better than leading algorithms. The ultimate goal of his approach, detailed in the 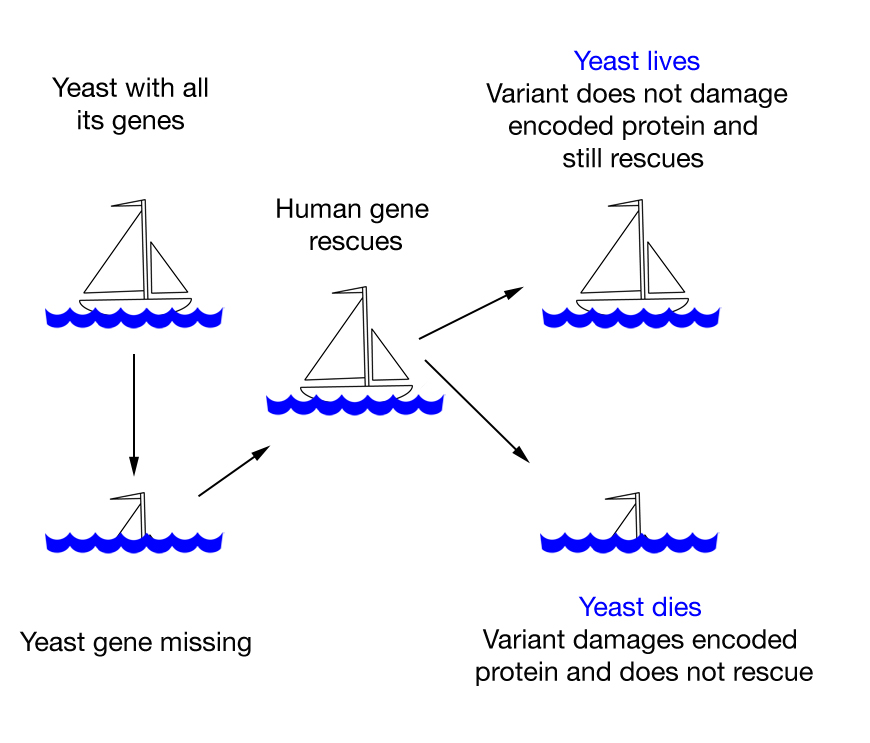 Yeast cells are simple, yet their basic architecture is similar to human cells. Because almost half of our genes have a shared ancestry with a yeast gene, it is often possible to study human genes in this easy-to-manipulate living organism.
Yeast cells are simple, yet their basic architecture is similar to human cells. Because almost half of our genes have a shared ancestry with a yeast gene, it is often possible to study human genes in this easy-to-manipulate living organism.