Main Second Level Navigation
Penney Gilbert
PhD

Qualification
- Stanford University, Stanford, CA, U.S., Research Fellow in Stem Cells and Biomaterials, 2007-2012.
- University of Pennsylvania, Philadelphia, PA, U.S., PhD in Cell and Molecular Biology, 2001-2006.
- Haverford College, Haverford, PA, U.S., BSc in Cell and Molecular Biology, 1995-1999.
MY RESEARCH OVERVIEW (GO TO SCIENTIFIC OVERVIEW)
Muscle stem cell bioengineering laboratory
Ah, the marvel of movement! Your skeletal muscle tissue is absolutely essential to your mobility, and your ability to breath, to swallow, or to blink. Each instance your brain decides it’s time to move, a signal is sent via your motor neurons to the neuromuscular junction where a burst of acetylcholine is released. This in turn actives a cascade of calcium within each skeletal muscle fiber invoking tiny molecular motors to crawl along cords of actin protein to produce a coordinated contraction of your limb muscle. As if this weren’t enough, when skeletal muscle is injured, resident adult stem cells are called to action to restore form and function.
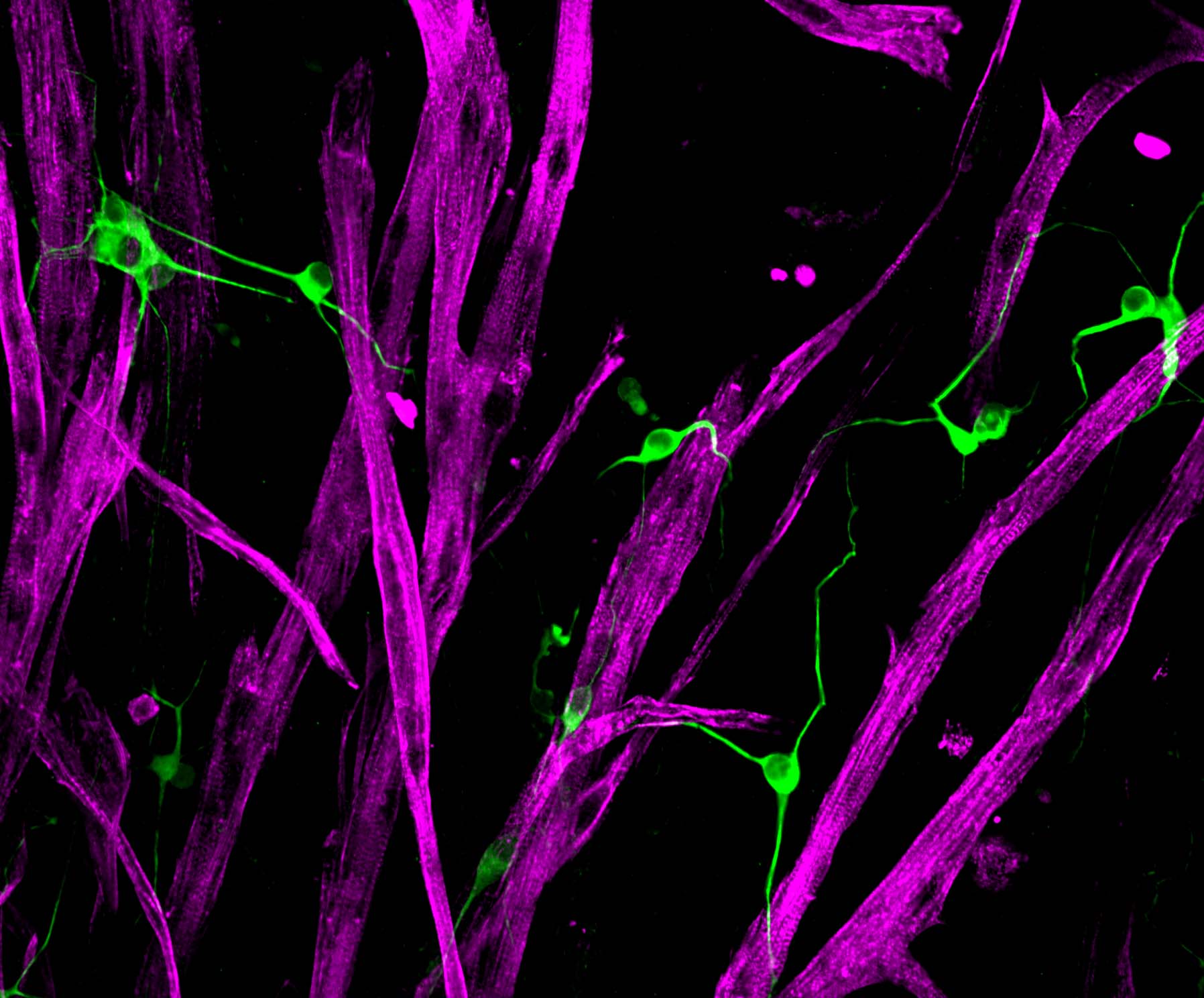
As we age, the ability of skeletal muscle to regenerate declines and the tissue becomes weak. Loss of skeletal muscle mass and function also occurs as a result of certain genetic conditions, inactivity and diseases like cancer. The goal of our research is to restore muscle function in these diverse disease settings by harnessing the potential of muscle stem cell biology.
To achieve our goal, we seek to identify cellular and molecular mechanisms underlying muscle stem cell niche regulation, which we test in our custom three-dimensional culture assays of healthy and diseased skeletal muscle. In the long-term, our research hopes to uncover molecular targets that can be targeted to augment muscle stem cell-mediated skeletal muscle repair.
Visit Dr. Penney Gilbert's Discover Research profile to learn more.
SCIENTIFIC RESEARCH OVERVIEW
Current projects in the lab include:
1. Muscle stem cell mechanobiology
Muscle stem cells, or ‘satellite cells’, are a rare population of cells in skeletal muscle tissue that sit on top of the long muscle fibers and beneath a blanket of proteins in their ‘niche’. For the most part, muscle stem cells are inactive, or ‘quiescent’, until they are called to action in response to tissue injury. It’s rather counterintuitive that a population of cells can remain quiescent in such a metabolically and mechanically active tissue like muscle!
We love to think about how physical features of the niche like tissue softness, topography, interstitial flow, or tensile strain influence satellite cell gene expression and their decision to remain quiescent or to divide and make more copies of themselves (self-renewal). Since these physical features change dynamically during regeneration and are altered in the course of aging and disease, our research program is poised to provide a unique picture of the biomechanical basis of skeletal muscle health and degeneration and identify novel therapeutic avenues to restore muscle strength.
2. Three-dimensional skeletal muscle models
Skeletal muscle is organized into a beautiful three-dimensional tissue. When the organization is disrupted, by injury or by other means, resident muscle stem cells are called to action to repair the damage and restore three-dimensional order. Using a histological approach on transgenic animal models, researchers capture a snap shot of the events that occur during tissue repair and the genes that mediate each stage. However, to date, few have watched skeletal muscle regeneration in real time, so our spatiotemporal understanding of the process is really quite limited. To overcome this challenge, we develop and characterize three-dimensional models of normal, injured and diseased skeletal muscle tissue. These platforms are enlisted to expand fundamental knowledge of muscle stem cell-mediated skeletal muscle regeneration and to support compound testing.
SELECT PUBLICATIONS
- De novo revertant fiber formation and therapy testing in a 3D culture model of Duchenne muscular dystrophy skeletal muscle. Ebrahimi M, Lad H, Fusto A, Tiper Y, Datye A, Nguyen CT, Jacques E, Moyle LA, Nguyen T, Musgrave B, Chavez-Madero C, Bigot A, Chen C, Turner S, Stewart BA, Pegoraro E, Vitiello L, and Gilbert PM. Acta Biomaterialia. 2021 May 20.
- Global and local tension measurements in biomimetic skeletal muscle tissues reveals early mechanical homeostasis. Hofemeier AD, Limon T, Muenker TM, Wallmeyer B, Jurado A, Afshar ME, Ebrahimi M, Tsukanov R, Oleksiievets N, Enderlein J, Gilbert PM, and Betz T.. eLife. 2021, Jan 18.
- A three-dimensional culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Bakooshli MA, Lippman ES, Mulcahy B, Iyer NR, Nguyen CT, Tung K, Stewart BA, van den Dorpel H, Fuehrmann T, Shoichet MS, Bigot A, Pegoraro E, Ahn H, Ginsberg H, Zhen M, Ashton RS, and Gilbert PM. eLife. 2019, May 14.
