Mobile Menu
-
Our Scientists
- Andrews, Brenda
- Angers, Stephane
- Attisano, Liliana
- Babaian, Artem
- Bader, Gary
- Blencowe, Benjamin
- Boone, Charles
- Brown, Grant
- Chan, Warren
- Fraser, Andrew
- Friesen, James (Professor Emeritus)
- Gilbert, Penney
- Gillis, Jesse
- Goeva, Aleksandrina
- Greenblatt, Jack
- Harrington, Lea
- Hughes, Timothy
- Kim, Philip M.
- Krause, Henry
- Montenegro Burke, Rafael (Rafa)
- Morris, John
- Morshead, Cindi
- Radisic, Milica
- Röst, Hannes
- Roth, Frederick (Fritz)
- Roy, Peter
- Ryu, William
- Sefton, Michael
- Shoichet, Molly
- Stagljar, Igor
- Taipale, Mikko
- van der Kooy, Derek
- Wang, Shu
- Wheeler, Aaron
- Yip, Christopher
- Zhang, Zhaolei
- Research
- Platforms
- Students
- Postdocs
- News
- Careers
- Events
- About Us



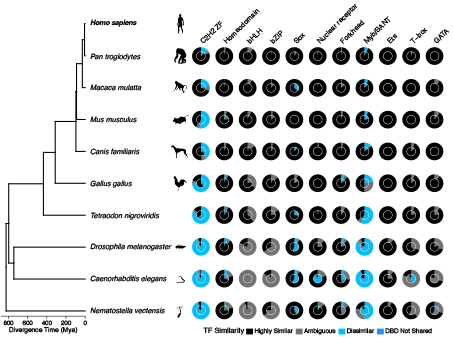 When Lambert compared all TFs across different species and matched to all available motif sequence data, he found that many human TFs recognize different sequences—and therefore regulate different genes— than versions of the same proteins in other animals.
When Lambert compared all TFs across different species and matched to all available motif sequence data, he found that many human TFs recognize different sequences—and therefore regulate different genes— than versions of the same proteins in other animals.