Main Second Level Navigation
Oct 8, 2021
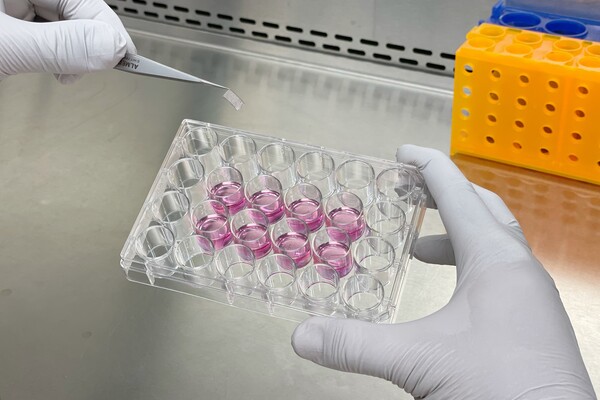
Donnelly Centre Investigators Develop Method for Testing Muscle Repair in Laboratory Dish to Impact Development of Cell Therapy
Research

Ting Yin
Sadegh Davoudi (left), a post-doctoral fellow, and Bella (Bin) Xu (right), a PhD student, both in the McGuigan and Gilbert labs are lead authors on a new paper that details their work creating a regenerative microenvironment in a dish.