Mobile Menu
-
Our Scientists
- Andrews, Brenda
- Angers, Stephane
- Attisano, Liliana
- Babaian, Artem
- Bader, Gary
- Blencowe, Benjamin
- Boone, Charles
- Brown, Grant
- Chan, Warren
- Fraser, Andrew
- Friesen, James (Professor Emeritus)
- Gilbert, Penney
- Gillis, Jesse
- Goeva, Aleksandrina
- Greenblatt, Jack
- Harrington, Lea
- Hughes, Timothy
- Kim, Philip M.
- Krause, Henry
- Montenegro Burke, Rafael (Rafa)
- Morris, John
- Morshead, Cindi
- Radisic, Milica
- Röst, Hannes
- Roth, Frederick (Fritz)
- Roy, Peter
- Ryu, William
- Sefton, Michael
- Shoichet, Molly
- Stagljar, Igor
- Taipale, Mikko
- van der Kooy, Derek
- Wang, Shu
- Wheeler, Aaron
- Yip, Christopher
- Zhang, Zhaolei
- Research
- Platforms
- Students
- Postdocs
- News
- Careers
- Events
- About Us


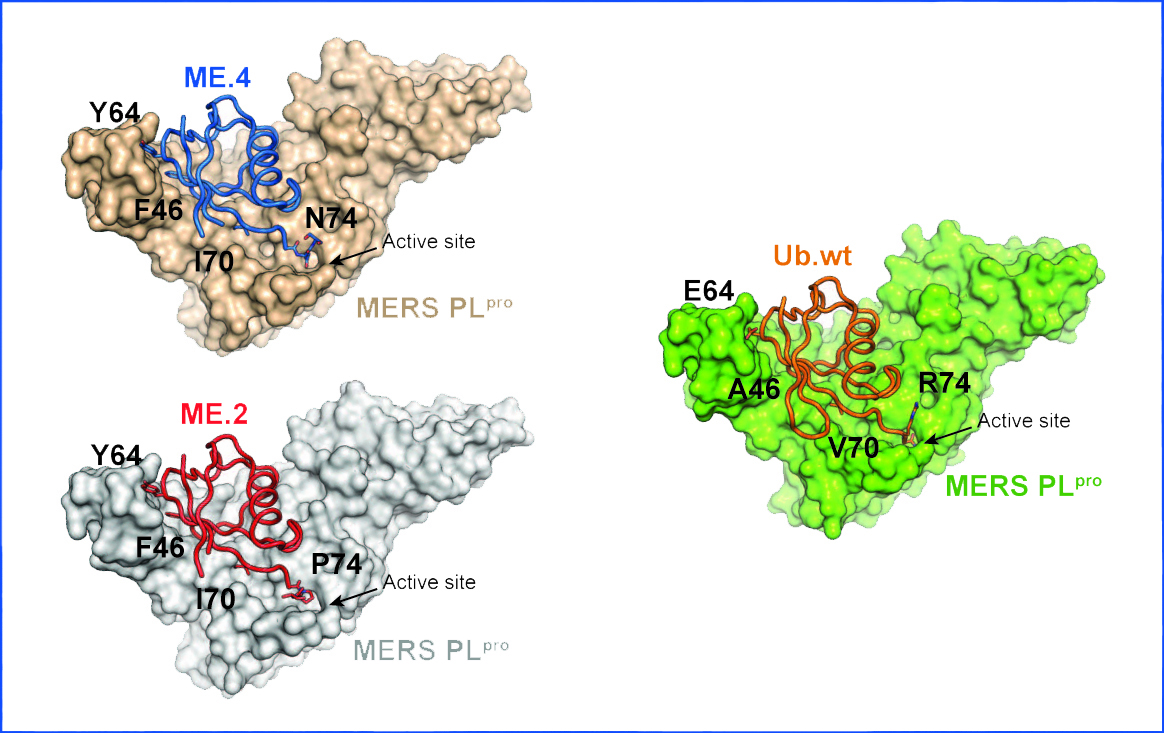 “Viruses have evolved proteins that allow them to hijack host proteins. We can now devise strategies to prevent this from happening,” says Zhang.
“Viruses have evolved proteins that allow them to hijack host proteins. We can now devise strategies to prevent this from happening,” says Zhang.